
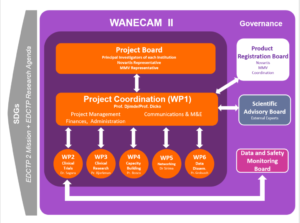
Our governance
A world without malaria.
Clinical development of a new combination of anti-malarial therapy, Ganaplacide/Lumefantrine for malaria control and elimination.
The objectives of WANECAM 2 are:
Meet Our Experts
Team bios
Meet Our Experts
WANECAM is the West African Network for Clinical Trials of Antimalarial drugs. This network is being supported by grants from the European & Developing Countries Clinical Trials Partnership (EDCTP).
The WANECAM 2 project aims to accelerate the development of the Ganaplacide/lumefantrine combination for the treatment of uncomplicated malaria by conducting clinical trials in four countries in West and Central Africa: Burkina Faso, Gabon, Mali and Niger. Moreover, and very importantly, the project includes activities around capacity building (e.g. training and infrastructure development) to improve the capabilities in West African countries to develop new antimalarial drugs. The aim is to advance the development of a much-needed new antimalarial therapy while strengthening clinical trial development capabilities in Africa.


A world without malaria.
Clinical development of a new combination of anti-malarial therapy, Ganaplacide/Lumefantrine for malaria control and elimination.
The objectives of WANECAM 2 are:
Meet Our Experts
Meet Our Experts